Chairman’s Reflections
Dr. Crooke is the founder, chairman and chief executive officer of n-Lorem Foundation.

As we near the end of 2025, we are deeply grateful for the trust placed in us by patients, families, and physicians. Together with our community of patients, physicians and supporters, we are demonstrating that individualized ASO medicines are not only possible, but are already delivering benefit to many patient families. While our work is limited by funding, our commitment to nano-rare patients and their families is boundless.
Our rigorous industrialized system has assured that each patient is treated with an optimal ASO, as demonstrated by the excellent safety and tolerability record and significant benefit that we have observed to date. We are particularly pleased that almost all patients treated for sufficient time have experienced significant benefit. We look forward to sharing much of this information with you at our upcoming Colloquium.
Each new patient requires an individualized ASO discovery and development effort that includes in vitro ASO discovery, animal toxicology studies, manufacturing and formulation in sterile vials ready for administration to patients. We have steadily streamlined and automated our ASO discovery processes so they maximize the value of every dollar we spend, and we are pleased that we have been able to work with providers of manufacturing, formulation of sterile vials and GLP toxicology to minimize those expenses as well. We benefit from the support of many contract research and manufacturing organizations. Nevertheless, for us to treat a patient today, it costs a little more than one million dollars and we have now received almost 400 applications.
We are thrilled that we have garnered such support that we have been able to grow far more rapidly than expected to meet the extraordinary demand we have experienced. Each year, especially this year, we have experienced greater success at achieving the necessary funding. This success gives us even more optimism that we will continue to be able to do the impossible with our patients and families and expand sufficiently to decrease the time before we can initiate activities that will advance ASOs for more patients. Nevertheless, we are not able to move as rapidly as is necessary today resulting in longer delays than we would like for many patients.
Our commitment to all nano-rare patients and families remains unwavering. We will continue to advance each program with the rigor, diligence, and scientific integrity that patients and their families deserve. As we have with all the challenges we have faced, we are optimistic that we can continue to fund our activities and increase our resources to meet demand. Our highest priority is to achieve sufficient funding to reduce the time from acceptance to treatment.
We acknowledge that waiting is difficult and want you to know that every member of our team is working tirelessly to respond to this extraordinary need. As we achieve even greater success at matching funding to demand, we hope to reduce the time between acceptance and when we can begin the discovery and development activities necessary to identify and develop an optimal ASO for each patient.
As we have with all the challenges we have faced, we are optimistic that we can continue to fund our activities and increase our resources to meet demand, and we ask the community to help us beat our goals by spreading the word about n-Lorem and connecting us to new fundraising opportunities.
We look forward to working with all interested parties to meet the extraordinary demand that we are experiencing and hope to see you at the colloquium in October.
With gratitude,
The n-Lorem Team
Dear supporters, partners, patients and parents,
As we move along into our sixth year, we have much to be proud of and much that we have learned, which we are now sharing. We also know that we have formidable challenges as we continue to grow to meet the ever-increasing demand and we are buffeted by the extraordinary political turmoil, as are so many institutions on which we all depend.
We have proven that what was impossible just a few years ago, can be done. Armed with ASO technology and the support of the FDA, we can initiate a new drug discovery program for each nano-rare patient and create a new personalized ASO medicine. We can do that repeatedly and we can provide these medicines to needy patients for free, for life — proving that “an impossible” non-profit model can work — and work well. We have proven that if you really understand ASO technology and industrialize the process as we have done, experimental ASOs can be administered to many patients with excellent safety and tolerability.
Most importantly, we have shown extraordinary benefit in 11 of the 12 patients who have been treated long enough to evaluate for benefit. We have shown benefit in patients with CNS, kidney, and eye diseases, and we have shown very long-lasting, profound benefit. We have proven that even patients suffering from the most advanced diseases can experience profound benefit. And we have shown that patients with profound developmental delays can recover long lost abilities or even abilities they never had. For the first time, we have proven that ASOs can dramatically reduce neuropathic pain and improve dysfunctions of the autonomic nervous system, the nervous system the controls heart rate, blood pressure, respiration, and the GI and GU tracts.

We have changed the lives of many patients and families and provided realistic hope to many.
We have built a world-class organization capable of filing more than 20 INDs a year. We have created systems and extramural review committees that help assure that we make the highest quality risk-benefit decisions for each of our patients. We have established a network of outstanding treatment centers with which we collaborate across America. We have proven that the novel approach we have taken to clinical trials is providing high-quality data and we are learning how to optimally use caregiver assessments. Finally, we have shared what we are learning through more than 10 scientific publications.
We have rapidly grown to meet the extraordinary demand. We received more than 350 Applications for Treatment, accepted more than 180 patients, filed more than 30 INDs, and are treating more than 30 patients. No organization has accomplished anything within 10 times of what we have done.
We have not accomplished all this ourselves. We have benefited from many industry partners, many generous donors, and many volunteers. We thank all of you for your support.
As I look to the future, I am confident when I say that we have overcome every “impossible” barrier, save one. Only continuing to raise the increasing funding needed stands in the way of treating thousands of patients around the world. As daunting as that challenge, I am buoyed by our success and confident that with your support, we will overcome the remaining challenge.
I am also proud to share that we will host our third annual nano-rare patient colloquium, Oct. 20 & 21 in Cambridge MA, where we will provide more details on what we are learning and what we can do together. I invite you to come to this important meeting. More details and free registration can be found on our website (www.nlorem.org).
Sincerely,

Stanley T. Crooke, M.D., Ph.D.
Dear Friends and Colleagues,
As we conclude 2023, though I know that we have many challenges ahead of us, I am ever more confident in the value of our mission and the future of n-Lorem. The confidence in our future is a product of the outstanding progress we have made this year, building on the momentum of our first three years, and I am pleased to share a few highlights with you in this letter while I extend best wishes for a joyous holiday season and wonderful 2024.
Delivering on our promises
We have now filed 10 INDs in four divisions of the FDA. We have treated six patients and hope to initiate treatments in the other patients shortly. As you know, even after the FDA has performed a thorough review of an IND, each institution in which a patient is to be treated reviews the treatment plan, the information that assures that treatment with the ASO designed for that patient is appropriate, and that review by the treating institution can take a number of weeks. Even more encouraging is the clinical experience as a number of our patients appear to be experiencing meaningful benefit from their ASO and the safety and tolerability are pristine, despite how progressed in their disease our patients are.
Equally important, we have completed the building, equipping and staffing of our main laboratory, which is now fully functional and delivering great results. This means that we can begin to make real progress in working through the many cases we have accepted for treatment and to initiate research activities that will improve the performance of our ASOs and hopefully increase the types of patients we can treat. To substantially improve the efficiency of our lab and accommodate growth, almost immediately after we completed the build-out of our lab, we are moving to a new, better, more efficient, larger lab, which, as a bonus, will allow us to consolidate all our San Diego team at a single site.
Sharing what we know and what we are learning
Knowledge and experience with ASO technology are critical requirements to provide effective and safe ASOs to nano-rare patients. Also important is real experience in drug discovery and development and it is vital to manage patients exposed to experimental medicines professionally. The n-Lorem team brings decades of ASO experience and a deep understanding of drug discovery and development to bear on the opportunities and challenges we encounter. It is important that we share what we know, how we identify optimized ASOs, our experience in the clinic and all that we are learning from nano-rare patients with all interested parties.
Because I believe that science is never done till shared with peers in a high-quality peer-reviewed journal, we emphasize scientific publications as a key venue in which we can share. We have now published eight peer-reviewed manuscripts, with clinical investigators have submitted case reports of nano-rare patients who have been treated and written a major paper describing the characteristics of the first 173 patients who applied for treatment. As that manuscript is in revision in a major scientific journal, I am optimistic that it will be published next year.
On the other hand, it is important that we share all that we know and are learning in a more timely fashion than peer-reviewed manuscripts. To that end, we have made hundreds of presentations. Most importantly, in 2023, we held our first nano-rare patient colloquium, which was generously hosted by one of our founding donors, Biogen. By all measures, that meeting was a great success. We presented what we do, how we do it, and what we are learning in detail and listened carefully to our patients and families and partners. Attendance was excellent and we are looking forward to our 2024 colloquium.
Working effectively with regulators
Throughout my career I have believed that if we put the needs of patients at the center and never waver from our commitment to do what is best for the patient, then couple that to a deep understanding and respect for the challenges of drug development, high quality science and transparency, regulatory interactions will be constructive. At Ionis, I led the creation of this technology and the establishment of a rigorous, transparent scientific environment. I believe that one of the reasons that the special guidance for ASOs for nano-rare patients exist is the reputation we established at Ionis. At n-Lorem, we have maintained that commitment and refined it as we think about the needs of individual patients. We continue to work effectively with the FDA because we share a commitment with them to help nano-rare patients while exposing our patients to only prudent risks. We will continue to do all that we can to earn the FDA’s respect and trust every day as we enter 2024 and beyond.
Creating the highest quality, most committed team possible
Our patients deserve the efforts of the highest quality team and it has been my goal to achieve that. But I worried that we might not be able to recruit outstanding leaders because we cannot offer the financial rewards of commercial companies. I am pleased to tell you that I needn’t to have been worried. The mission is the only recruiting tool I have needed and, in 2023, we completed building the senior leadership team, the mid-manager team and the laboratory team and every position is held by an outstanding person. I am confident when I say that I have never had a better, more committed, more cohesive team than I have at n-Lorem. We focus our outstanding team on meeting the unique needs of each of our patients and the quality of the team is displayed in every ASO for every patient.
Taking center stage
n-Lorem is unique in vision, scale and quality. Thus, a major goal in 2023 was to assure that n-Lorem and our unique qualities were broadly known and understood. We think we achieved that in 2023. The growth in the popularity of our podcast series, the success of our nano-rare colloquium, our national media coverage and our social media presence are evidence of that success.
Fundraising
2023 was another successful year in fundraising. We exceeded our challenging fundraising goals again this year and opened multiple new funding sources as well as continuing support from past donors. We enter 2024 with numerous opportunities we are pursuing. Of course, the more we succeed, the more funding we need, but I do see a path to sustainable financing, and we are ahead of schedule in achieving that goal. Once again, the primary reason I can say that is the rightness and power of our mission. That said, we will need your help and every dollar matters.
2024 and beyond
We enter 2024 with great momentum and optimism. We will be able to do more and do it better. Please join us as we do our best to change the world for the better, one patient, one family at a time.
Sincerely,

Stanley T. Crooke, M.D., Ph.D.
Dear supporters, partners, patients and parents,
As we enter 2023, all of us at n-Lorem know that this next year will be demanding and intense as we attempt to respond to the overwhelming demand and raise sufficient funds to support the added growth in the organization that must be achieved if we are to respond to the needs of our patients. While being intensely busy simply trying to advance ASOs for as many patients as we can afford, a major effort is underway to streamline our processes, thereby saving dollars and time and seeking solutions that will enhance the number of patients we can treat this year and beyond.
I am also quite excited that the n-Lorem team is writing several highly informative manuscripts that reflect the tip of the iceberg of what we have learned to date. As publication of scientific data in quality peer-reviewed journals is a slow process, we also plan to share key learnings through our podcast series. Importantly, the entire library of podcasts and supplemental materials for listeners is located in a new, far more informative and useful n-Lorem website.
In this annual letter, I discuss the progress we have made, but also revisit some of the most basic concepts that underlie all that we do at n-Lorem. Finally, I will provide a high-level summary of our plans for 2023.
Every Patient Matters
A core belief at n-Lorem is that every patient matters. The first dictum of therapeutics is to treat the patients who can be treated today while investing long term to identify better solutions for more patients. We know that n-Lorem cannot address the needs of all patients, but that can never justify failing to provide treatments to those who we can help today. We are committed to enhancing health care and changing the world one patient, one family at a time.
I am confident that all human beings share the view that if we can help a patient today, we have a moral imperative to do so. However, when there is a beloved family member who is suffering, it is difficult to watch other patients be treated while a loved one is not a candidate for treatment. That is an understandable emotion, but I am sure that as caring people contend with these justified emotions, they will find a way to be happy for those who may benefit from treatment while supporting those seeking new therapeutic approaches that, in the long run, may benefit ever more patients.
Promises
Every new patient represents a novel ASO discovery and development program. The essence of science is uncertainty and the process of discovering a novel medicine is complex and arduous, even for ASOs. Given this ambiguity, we can make only two promises:
- We will work as hard as possible to provide an optimal experimental ASO.
- We will assure that the clinical exposure of each patient to an experimental medicine is managed professionally by scientists with drug discovery and development experience. Every patient deserves a quality ASO that maximizes the opportunity for benefit while minimizing the risk of side effects.
Blazing New Ground
What n-Lorem is doing has never even been considered possible until the technology that was developed by Ionis Pharmaceuticals under my leadership was invented, advanced and validated. A non-profit model is the only solution available today for the rarest and most unserved population, nano-rare patients. n-Lorem is pioneering the industrialization of the discovery, development, manufacturing and provision of personalized experimental ASOs for free for life. We define nano-rare patients as those individuals who have a single gene mutation that is expressed in no more than 30 patients worldwide. Why 30 patients or less? Because there is no commercial path for such a small group of patients, and we believe that we can raise sufficient funds to provide an experimental ASO for free for life to this rarest and neediest patient population.
Though we are encouraged by the support we have received to date, the demand has outstripped all expectations and the funds we have been able to raise. Our immediate challenge is to raise sufficient funds to expand to begin to meet today’s demand, then show that our model is sustainable. These are formidable challenges, but we know that n-Lorem is the best hope for these unserved patients.
Response to Demand
As I have discussed in previous communications, the demand for experimental ASO treatment has exceeded all of our expectations. In fact, at the close of 2022, we had received more than 180 applications for treatment and had accepted more than 80 patient programs for treatment. We anticipate the demand to continue to grow significantly in 2023. In response, we have expanded far more rapidly than planned. The senior leadership and next level leadership teams are now fully staffed. Every individual we have added is experienced and has a track record of success in their area of expertise and all are cohesive and highly effective, despite the newness of the teams.
We have opened a new facility that includes a well-equipped laboratory that more than triples our capacity to discover and develop experimental ASO treatments for nano-rare patients. We expect to expand our basic research and ASO discovery and development activities significantly beyond where we are today as we get our new lab up and running. In 2023, I plan to lead basic research and advance the technology with the goal to tackle some of the more challenging ASO patient programs and enhance the performance of our ASOs.
One of my major concerns as I founded n-Lorem was that it might be difficult to recruit truly talented experienced scientists, physicians and other key members of our team since, as a non-profit, we cannot match the salaries of for-profit companies. Nor can we provide the stock options or restricted stock units (RSUs) that corporations offer. I am gratified that the reason so many talented people have joined us is the mission.
Importantly, in about two weeks in August of 2022, we filed four Investigational New Drug (IND) applications. INDs are the regulatory documents required before treatment of a patient can begin, and these INDs were submitted to two different divisions of the FDA and were rapidly approved. As a result, we are now treating patients and will be filing many more INDs this year. In fact, our minimum goal is to file at least 10 new INDs, but if we can continue to generate sufficient financial support, we have the capacity to double this number in 2023.
Communication
Since inception, we have published a number of peer reviewed manuscripts, given hundreds of presentations in which we described our mission, our quality processes and described each step in the screening process we use to identify the optimal ASO for each patient and issued scores of press releases. Though we cannot guarantee that there will be no side effects, we are committed to exposing the very sick patients who we treat to only the most prudent of risks. Importantly, as we learn more, we continue to refine our screening process to shorten the time and reduce cost while assuring the most optimal ASO possible.
Our podcast series has been very well received and, new this year, we will leverage this communication tool to reiterate our drug discovery and development process in detail. Although we have communicated this process before, we think that it is so important for all interested individuals to understand the quality processes we have created, why each step in the creation and evaluation of an experimental ASO is critical, what is learned in the identification of an optimal ASO and why it is so important. As such, we will augment our current interview and science lectures with a podcast series where we will discuss in detail each step in the n-Lorem process. We will describe how each activity contributes to the time and cost of developing an optimal ASO and how important quality and ensuring only the most optimized ASOs are developed. All ASO programs present unique challenges and in this series, I also will highlight several programs that have presented significant time delays as illustrations of what can introduce extended timelines into our programs.
Empowering Patients
I am also proud our progress in empowering and educating our patients, parents and other interested parties. Our podcast series consists of a mix of interviews and science “lectures” that provide a basic understanding of health, disease and treatments beginning with what a chemical is. The audience for our podcast series continues to grow and we have many exciting and informative podcasts that will be coming your way in 2023. In fact, we are adding two new podcast formats, the podcast I discussed above and a quarterly Q&A podcast.
Social Media
Additionally, we have strengthened our presence on social media. Recently, for example, on my LinkedIn page, in the “Let’s discuss science” series, I provide a guide as to how to think about possibly drug side-effects, “How to think about drug related toxicities”, that you might find of value. Lastly, we have been working for some time to create a much more informative and accessible website that we launched earlier this month.
Another important type of communication concerns the status of specific ASO programs for specific patients. Not surprisingly, we are asked by patients and parents frequently about the potential for success in identifying an ASO and the progress and timing of when an ASO will be available to treat that patient. As a general rule, we think that such communications should be between the treating physician and the patient or parent. When appropriate, we provide information to be discussed directly to the treating physician, who will continue to communicate with the patient or parents.
We will continue to try to optimize our approaches to communication and look forward to your comments and suggestions.
Health Equity
n-Lorem exists because we believe that all patients deserve the opportunity to be treated, irrespective of the rarity of the disease or the economic status of the patient or family. If we can prolong and enhance the life of a patient and return hopes a dreams to a family, we are morally obligated to do it. We focus on patients who have diseases caused by a single gene mutation that is found in no more than 30 patients in the world. We choose this patient population because today there is no path to commercial approval or commercial model that can address the needs of such rare patients and because we hope that we can raise sufficient donations to treat as many nano-rare patients as we are able to.
We are industrializing the treatment of nano-rare patients because we believe that each patient deserves the very best ASO that we can discover and develop and the professional management of the exposure of the patient to an experimental medicine. We have established quality processes that assure that each patient is treated with an optimal ASO. Our process begins when an application for treatment is received. The application is blinded, meaning that we have redacted all personal details on the patient. When evaluating an application, the only factors considered are the prevalence of the mutation, the feasibility of treating the patient with ASO technology, the status of the patient, the rate of disease progression and the availability of a research physician committed to treating the patient and evaluating the performance of ASO at an institution capable of and committed to the treatment of a patient with an experimental medicine. The economic status of the patient is not considered. Nor is the question of whether the patient or family is a donor considered. Unless a patient or parent contacts n-Lorem or discloses the identity of the patient publicly, we are blinded to the identity of each patient.
Treating Patients With the Rarest of Mutations Is A Moral Imperative And Treatment of One Will Benefit All Patients
Though many of our patients have mutations that cause illnesses that have never been identified or named, we now know that for almost all rare genetic diseases, there are typically one or two mutations that account for most of the afflicted patients and multiple extremely rare mutations that may cause disease in one to a few patients. On occasion, for patients with a known and named disease and a patient advocacy group, we are asked how the treatment of one or a few patients with the rarest of the mutations that cause the disease helps those who are not candidates for n-Lorem provided experimental ASOs. Our answers to these questions are described below.
- Why Treating One Patient Benefits All Patients With A Rare Disease
As I discussed above, the most compelling reason to treat a patient is that every patient matters. As we age, we learn that what were black and white tenets are many shades of gray. Not so for the belief that every patient matters. I say this with absolute moral certitude that has informed my life and career and is why n-Lorem exists. That said, though there are other compelling reasons to treat even one patient in a population of patients with the same disease, but perhaps not the same mutation. - All Knowledge Matters
Advances in science are incremental and each additional bit of knowledge contributes to the evolution of understanding. No area of scientific inquiry is more incremental than advances in the treatment of diseases. What can be learned by addressing the needs of patients with the rarest of mutations that cause a more common rare genetic disease is immediately applicable to the entire group of patients with the disease. There is no better way to learn about the molecular causes of a disease and potential value of therapeutic interventions than studying a patient or a few patients, even if they have the rarest of the mutations known to cause the disease. - Acquiring A Corporate Partner To Commercialize A Medicine For The Entire Disease Population
When a biotechnology or pharmaceutical company must make a development decision, even for a rare disease drug, the investment is likely a minimum of $100 million. The key problem at the time such decisions must be made is that the intrinsic failure rate of drugs at that stage demands that any future value must be discounted by 80-90% and then the economics simply do not work for any reasonably sized company. The hard truth is that there are simply better ways to invest shareholder money. This means that products for small patient populations are usually not developed commercially and today, this basic math is exacerbated by the fact that current pricing of rare diseases drugs is under pressure. Unfortunately, the best way to reduce risk meaningfully is to obtain positive clinical data in a few patients with a disease. n-Lorem is uniquely positioned to treat patients with the rarest of mutations known to cause the disease and determine if the experimental ASO appears to be beneficial.Further, the leadership of n-Lorem has decades of experience in partnering in the drug discovery industry and is committed to using the data to find a responsible commercial company to develop a medicine for the disease in question. In fact, there is already an example of the success of a personalized ASO encouraging full commercial development for a rare disease. - Industrialization of the Treatment of Nano-rare Patients
I have emphasized how vitally important it is to work with scientists and physicians thoroughly knowledgeable and experienced with ASO technology to assure that patients are exposed to only optimal ASOs. Additionally, I have also spoken and written repeatedly about how vital it is to assure that the clinical exposure of patients to experimental medicines of any type be managed by those experienced in managing the development of experimental medicines. To my mind, these concepts are so self-evident as to be unworthy of discussion. Just as it would be foolish and dangerous for me, even though I am an M.D., Ph.D. with house staff training in internal medicine, to take care of a child with a complex neurological disease, it would seem equally foolish and dangerous for a physician who has never contributed to the basic advancement of antisense technology or developed a new drug, to try to discover and administer an optimal ASO. In short, I have warned of the dangers posed by “amateur ASO makers” repeatedly and offered to collaborate with any competent clinician. Gratifyingly, almost all academic physicians have chosen to work with n-Lorem.
Conclusions
n-Lorem is off to a strong start, but we are not even at the end of the beginning of the journey to bring hope and help to patients with nano-rare mutations. I am pleased that the quality systems we developed are working, I am thrilled to be treating multiple patients with many more INDs coming and I am proud of how hard we have worked to communicate about what we are doing. However, we know we can to more and do it better. We are looking forward to the even more effective approaches to communication approaches that I mentioned and to enhancing many elements of the processes we have created.
In the next installment of my annual letter, I will focus on our plans for 2023 and beyond and the exciting things we are learning. I look forward to serving many more nano-rare patients and sharing all that we learn with you.
Sincerely,

Stanley T. Crooke, M.D., Ph.D.
Dear supporters, partners, patients and parents,
As we enter the second half of 2023, I hope to take a moment of your time to thank you on behalf of our patients for your wonderful generosity and to provide a summary of all that we have accomplished in three and a half years. I am proud of what we have accomplished but know that the need is so great that we must re-double our efforts to meet the needs of the nano-rare community.
Treating patients in need today
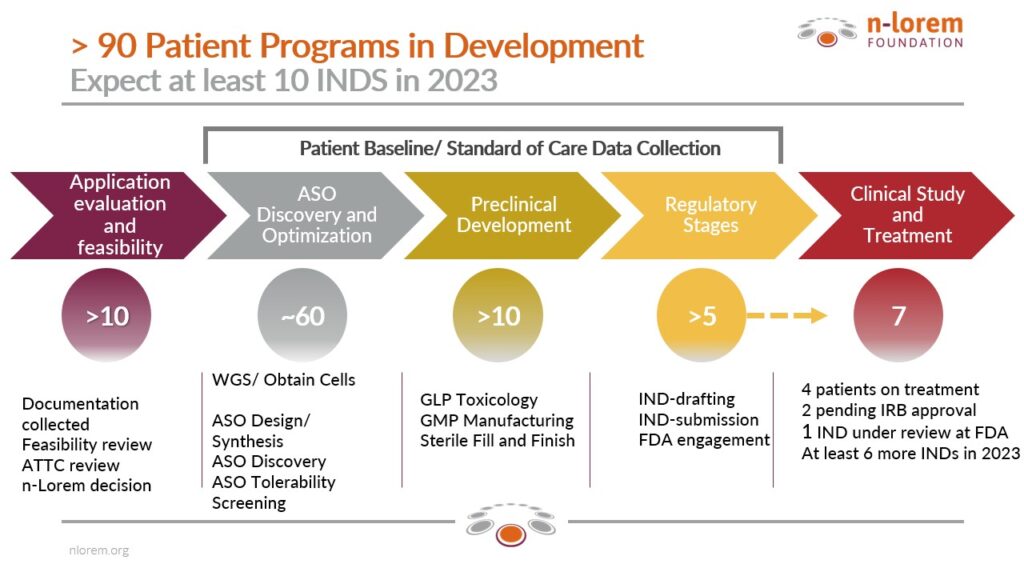
Because the demand continues to increase, despite all the resources we have added, we are still not able to get treatments to patients as rapidly as we would like. We have now processed applications for the treatment of more than 200 patients and accepted about 100 patients for treatment. Our current pipeline of hope and potential help represents an extraordinary achievement of a tiny group of committed, caring professionals. To have filed seven investigational new drug applications (INDs) with the FDA in so little time with such a small organization is a stunning achievement. Each of these INDs represents a needy patient and a desperate family. Importantly, we have had INDs approved by three different divisions of the FDA, meaning that patients with diseases in the brain, the eye and the kidney are potential beneficiaries. Our pipeline shows that we are advancing patient programs across all areas of discovery and development.
Evidence of benefit
Given how advanced the diseases are of most of our patients, I worried that we might not be able to provide meaningful benefit to any patient. Well, I no longer worry about that. The experience with Anna and, most recently, Susannah give us a resoundingly positive YES to the question if patients with advanced diseases can be helped. This is great news for all patients and Anna and Susannah are true pioneers showing the way for patients to be treated with personalized ASOs. To have such clear observations of help so soon is deeply rewarding to all of us involved in this effort and provides hope to those awaiting treatment.
Nano-rare patients deserve the quality at every critical step leading to treatment
I am pleased to report that the quality systems we implemented on day one are doing the job they were designed to do. From the moment we receive a patient’s application, we understand that we have a patient’s well-being in our hands. We are delivering quality, especially the all-important steps of assuring that each patient is treated with an optimal ASO that only exposes the patient to prudent risk.
Health care equity
In a developed economy, I believe access to quality health care is a right, and at n-Lorem, we do our best for every single patient every single day. We do take what we call ‘directed donations’ from groups and individuals interested in supporting research on a particular gene or mutation. These directed donations support and expand our infrastructure, which supports the treatment of all patients. For each directed donation, we request a donation to our general fund to accomplish this. Importantly, what we learn from research on one gene informs what we know about all nano-rare mutations. It is impossible to overemphasize the long-term value of what we learn from each gene we study and each patient we treat. Long term, I am confident that what we are learning will benefit all humans.
Meeting the demand
Most importantly, we have expanded to try to meet the overwhelming demand. As our new lab comes online, we will more than triple our ASO discovery capacity. Our ability to build, equip and staff our new lab happened years earlier than planned because of your generosity, our supporters.

But a lab is a shell and what makes it exciting and productive is the quality and commitment of the team in the lab. I am thrilled with the team of dedicated scientists we employ at Ionis and La Jolla Lab and those who have joined us in our El Cajon laboratory (pictured below).

Building a senior team that nano-rare patients can count on
I have been running organizations for many years and can state with confidence that the senior team at n-Lorem is the best team I have had the privilege to lead. We are all united by our mission and we believe that all patients matter. No patient should be left behind. The senior team is now supported by an equally capable more junior team.

Sharing what we learn
We must share what we learn as broadly as possible. At n-Lorem, we have already published 8 peer-reviewed manuscripts in high-impact journals. In the latest paper in Nature Biotechnology, we describe the plight of a single one of our patients, then lay out the reforms so desperately needed, and will be submitting additional publications this fall. I am absolutely certain that these reforms are not only affordable but will reduce the cost of health care over time. We need every one of you to put your shoulder to the wheel here and help us drive the reforms through state and national governments and the insurers who you pay every day for your health care insurance. Make all of these folks listen and join us in this effort that will help all patients.
On October 12, we will welcome all to our first n-Lorem Nano-rare Patient Colloquium, a collection of patients, partners and physicians. We are tremendously excited to have the opportunity to share what we have accomplished what we have learned and the challenges we face with all interested parties. We look forward to seeing you there. Our wonderful donor and partner, Biogen, is hosting this gathering and I am excited to tell you that Biogen’s CEO, Chris Viehbacher will join us to express his commitment to nano-rare patients.
Creating a community
I am thrilled with the response to our Patient Empowerment Program. If you haven’t listened to both the interviews and the informative lectures in our podcast series, I hope you do. I think you will enjoy them, learn from them and emote with others who suffer from nano-rare mutations.
We can do this!
I was told that helping nano-rare patients today was impossible. Thanks to your support, I can say that it CAN BE DONE TODAY, and with your help, we will do more tomorrow.
Gratefully,

Stanley T. Crooke, M.D., Ph.D.
Dear supporters, partners, patients and parents,
I hope that all of you have had a wonderful and joyous holiday season and send you my very best wishes. Like most of you, I am asking myself how 2021 passed so quickly. I also marvel at how rapidly the first two years of n-Lorem have passed. On behalf of the patients we serve, I thank each of you for your interest and support. We at n-Lorem know that we could not have achieved the remarkable progress I share with you today without your support. I also extend a heartfelt personal thank you for the privilege of being a part of the solution for nano-rare patients.
More than four decades ago, I made the difficult decision to focus my efforts away from the day-today care of patients to building leading pharmaceutical and biotechnology organizations where I have been able to have a broader impact. During this time and in addition to my leadership and management responsibilities, I continued to advance the scientific understanding of RNA as an active scientist. As I reflect back today, I think that was the right decision, but I have missed directly caring for patients since. For me, the intimacy of responding to the needs of patients one patient at a time feels like practicing medicine again. Thank you for this privilege. I wish that I could promise every patient that we will succeed in our goal of creating an ASO medicine that improves their health, but of course, I cannot do that. Each patient represents a new drug discovery challenge. What I can promise is that I and the team I have assembled are working as hard as we can every day to serve the terribly underserved nano-rare patients who we can help. I hope that you will agree with me that the progress we have made in our first two years suggests that we are very much on the right track.
In parallel with establishing n-Lorem, we have had the privilege to work with Dr. Neil Shneider and to treat 12 patients with a rare aggressive form of amyotrophic lateral sclerosis (ALS). Patients with this aggressive form of ALS have a mutation in the FUS gene and typically experience symptom onset early in life, rapidly progress with loss of muscle function and usually succumb to their disease within months or perhaps a year after symptom onset. Our experience with these patients is very important as they represent the largest series of patients treated with personalized experimental ASOs to date and support the viability of our mission. We are encouraged with the data we are observing in these patients who are being administered experimental ASO medicines. We look forward to sharing these data with you when Dr. Shneider publishes a summary of his experience. We recently had the opportunity to speak with one of Dr. Shneider’s patients and her family. I want to share a quote from Dr. Shneider with you about his experience.
“The program began as an effort to help a single person from a family already devastated by this rare and aggressive form of ALS. Unexpectedly, the n=1 program grew to involve the expanded access treatment of a dozen ALS-FUS patients, and ultimately, to a global phase 3 trial of this antisense therapeutic. I am hopeful that we have had a significant impact on the course of disease for our patients, but what I am certain of is that we have had an impact on the lives of these individuals and their families, offering hope that derives from the potential for benefit. It is a meaningful gift, one which n-Lorem offers to many whose lives and loved ones are threatened by these rare, but still too common disorders.”
Neil Shneider, M.D.
The Roles of n-Lorem
A central dictum of therapeutics is to treat the patients who can be treated today with the technology available while investing long term in research that can advance and broaden treatments for all patients. I think that n-Lorem is playing a key role in both the short-term opportunity to help the patients we can help today and the long-term effort to create better more holistic solutions for nano-rare patients in the future.
Today, most patients with diseases caused by nano-rare mutations progress and succumb to their disease without having a genetic diagnosis. These patients never understand why their lives are shorter and more limited than others. With the advances in genomic sequencing and the efforts of numerous personalized medicine centers in tertiary care medical centers, the tools exist to be able to genetically characterize any patient. However, thousands of patients who have been fortunate enough to undergo a genetic diagnosis are informed that there is no treatment, and worst of all, it is unlikely that there ever will be a treatment for them.
n-Lorem is proving that there is hope and there can be a personalized treatment for many of these patients. While we cannot help all patients, we can and are helping many. The opportunity for ASO treatment provides hope to the hopeless and eventually, we hope, treatments for many. Hopelessness and despair that come at the end of a long and perilous journey to diagnosis are devastating.
This is n-Lorem’s primary, short-term task, but we are progressing in other efforts that will add value in the short-term. We have been an effective voice in the growing chorus of concerned stakeholders to bring the plight of the nano-rare patient to center stage, and we will continue to do that. Additionally, we know that nano-rare patients are isolated and frustrated and at n-Lorem, we are developing a portfolio of patient support systems, including a podcast series that will focus exclusively on the nanorare patient. In this podcast series, we will interview a broad range of stakeholders to assure that all voices have a platform and intersperse the interviews with a series of ‘lectures’ intended to provide the intellectual framework that will support a deeper understanding of the nature of the syndromes and diseases that nano-rare patients suffer from, how drugs work and how ASO technology enables what would have been inconceivable until now: the discovery and provision for free a personalized ASO treatment one patient at a time.
The longer-term roles n-Lorem hopes to play are also important. As we pioneer an entirely novel approach to providing treatment to many nano-rare patients, we blaze an entirely new path for others to follow as other technologies mature and can be brought online to treat these patients. The leverage of blazing a new path and creating a model of quality throughout the process is the key to advancing from treating thousands of patients to millions. Furthermore, the opportunity for treatment will spur efforts to assure earlier genotypic and phenotypic characterization of nano-rare patients. Ultimately, genomic sequencing must become a standard approach to newborn health assessment. Only then will the true incidence and prevalence of nano-rare mutations be understood, and only then will it be possible to treat patients before they advance to more serious manifestations of the mutation.
Responding to the Overwhelming Demand
Upon forming n-Lorem, I expected the demand for ASO discovery and development to be limited and increase slowly. Given that expectation, n-Lorem was staffed almost entirely by volunteers, many of whom were part-time. I assumed that n-Lorem would have three to four years in mature from a volunteer effort to full-time paid staff. However, the demand exceeded my expectations by 10-20 fold.
In response, we have moved rapidly to strengthen the senior team to set up the foundation to manage much more effectively.
- Sarah Glass, Ph.D., Chief Development Officer
- Joseph Gleeson, M.D, Chief Medical Officer
- Tracy Cole, Ph.D., Director of Research and Development
- Amy Williford, Ph.D., Director of Communication
We have also expanded the laboratory team and added several scientists to the laboratory to enhance ASO discovery and are also implementing a number of approaches that should lead to enhanced efficiency and better management of the ASO discovery process. We have taken on many more activities that were provided by volunteers as we simply outgrew the ability of volunteers to meet the needs of our patients. Despite all these investments, we are not meeting our goal of treating patients within 15-18 months of acceptance of an application. We will expand our laboratory staff further and continue to seek solutions to catch up to the demand. Then we hope to meet our aggressive treatment goal.
We thank all of our volunteers for their strenuous efforts and assure our patients that we are
working as hard as possible move ASO treatments forward as rapidly as possible.
Strengthening the n-Lorem Team
In addition to greatly strengthening our senior leadership team, we have added key elements necessary to assure quality performance from application to treatment. The evaluation of the performance of the ASO treatment requires the choice of service providers and key partners. In our first year, we established relationships with the key ASO manufacturers, contract research organizations that perform preclinical toxicology studies, the UDN and many other providers.
This year, we have built upon our earlier successes. Some highlights include:
- Selecting Parexel as our clinical partner. Since we envision treating thousands of patients and managing 100-150 individual natural history studies and clinical studies simultaneously, the selection of a partner to help us with these enormously complex tasks and build and manage a database that we can analyze to assess our overall impact on the lives of our patients, making the right choice here was critical.
- Strengthening our Board of Directors, by adding Jason Wood and Gene Yeo Ph.D. Jason brings extraordinary financial expertise to our board and Gene brings a deep understanding of RNA and broad experience to our collective intellect.
- Adding John Maraganore, Ph.D, as chairman of the Advisory Council was a vital step as John brings great knowledge and experience in RNA-targeted drug discovery, broad business leadership experience and financial expertise that will help us in many areas, including fundraising.
Regulatory Guidance
Nano-rare patients present such unique challenges that it is not possible to consider any modification of traditional approaches to the commercial development of a medicine. Just as the challenges demand a non-profit model and a drug discovery novel technology coupled to academic personalized medicine centers, the unique challenges presented by these unique patients require a specific and unique regulatory approach.
Consequently, we are grateful that the FDA expeditiously has issued guidance specifically for ASO treatment of nano-rare patients.
- January 2021, administrative guidance
- April 2021, preclinical guidance
- December 2021, clinical and manufacturing guidance
These earlier guidances provided clear and limited requirements for preclinical testing of ASOs for nanorare patients and provided a path for nano-rare patients to access treatment that is affordable and can be completed relatively rapidly. Given that most of these patients have advanced progressive disease by the time they are presented to n-Lorem, it is vital to able to respond as rapidly as possible.
Then, in December 2021, more ASO guidances were issued by the FDA that provided additional information regarding both the manufacturing processes and stability testing required for ASOs to be administered to nano-rare patients and the clinical administration of ASO therapeutics for nano-rare patients. We are grateful for the expeditious response to the needs of nano-rare patients reflected in the guidance. Also, important to consider is that the FDA’s guidance establishes a precedent for other regulatory agencies to consider when we extend n-Lorem into other countries.
Broadening Awareness of n-Lorem
Over the first two years of n-Lorem, a central goal has been to assure that all relevant audiences are aware of the opportunity that n-Lorem presents to provide therapy to some nano-rare patients today. Though we have made great strides and I believe that n-Lorem is seen as a leader in addressing the challenges of nano-rare diseases, we have much more work to do. Our most important task has been to make the patient and physician communities we may serve aware of the opportunity that n-Lorem presents to treat patients today. Based on the demand, we have clearly been more successful than anticipated, but there is still important work to finish.
In 2022, we must assure that the liver, kidney, in-born errors of metabolism and ophthalmological physician and patient communities are as fully informed and energized as the neurological communities.
We have also made meaningful progress in assuring those potential contributors and donor are aware of n-Lorem with numerous interviews, podcasts and presentations. We have also begun to experience broader lay media interest. Once again though, we have much more work to do. Having brought on broad experienced communication expertise and added media consultants, I am confident that the larger communities of interest will be fully informed within the next year or so. There is also interest in n- Lorem in the E.U. and Asia, but I think it is important that we be ready to meet the needs of patients in those areas before we invest in too much communication in those areas of the world, and it will take some time and effort before we are ready to expand beyond the U.S.
Strengthening and Broadening the n-lorem Network of Stakeholders
The challenges presented by nano-rare patients are far too complex for n-Lorem to solve even a fraction of the issues without support. Thus, our goal from inception has been to build as broad and effective a network of contributing stakeholders. I am deeply grateful for the responses to our requests for support. In 2021, we added a number of new partners including:
- Two new biotechnology companies, Alexion and Alnylam to our existing partners, Ionis, Biogen and Ultragenyx.
- Cytiva provided support for our ASO development and manufacturing activities by generously contributing equipment and supplies.
- Argonaut to manage sterile preparation of the vials that are being used to treat our patients.
- Parexel as our partner in the management of clinical trials and data management.
With these additions, we have completed the creation of the supply chain from application to treatment and assessment of the impact of ASO treatments on patients. This is a critical step in assuring that our patients get the very best quality possible at every step and that we maximize learnings from each patient and our aggregate experience. We also continue to add new tertiary centers that are providing the necessary genetic and phenotypic characterization of patient and the referral of those patients to n-Lorem for potential treatment.
Fundraising
Entering 2021, I believed that fundraising in our second year would be quite challenging because we would no longer be “the shiny new” thing and Covid 19 was clearly going to be meaningful impediment to fundraising. Additionally, advancing conversations with potential larger donors consume a good bit of time and multiple interactions.
Nevertheless, we exceeded our fundraising goal by more than 50% and added a number of new donors and partners, despite having limited opportunities to meet potential donors in person. Equally importantly, discussions with multiple potential partners and donors seem to be progressing nicely.Thus, I end 2021 even more confident that we will be able to demonstrate that a non-profit model is sustainable.
Patient Support
One truly is “the loneliest number” and most nano-rare patients are exactly that: the single patient in the world with their unique mutation and disease. Whether a nano-rare patient is truly n-of-1 or a member of a tiny patient population, the patient is entirely isolated. The patient or parents will have spent frustrating years bouncing from physician to physician with the hope of achieving a diagnosis while the manifestations of the disease worsened, a perilous and isolated journey that typically ends in hopelessness and there is no other person to ask what’s next, what can I expect? In response, we are developing an array of pertinent support efforts that will seek to create a sense of community for nanorare patients that will include a podcast series that will focus exclusively on nano-rare patients and include a variety of interviews of stakeholders and “lectures” that will try to fill the knowledge and information void about the nature of nano-rare mutations and diseases, how drugs are discovered and developed and how to think about drugs in a more sophisticated fashion. We hope to fully implement our patient supports systems in 2022.
Patient Support
n-Lorem has recorded two outstanding years with substantial progress across the board. We have also learned a great deal and much of what we have learned has come from chatting with patients and parents who present unique opportunities to learn from each individual and extrapolate to conclusions that can be extended to general conclusions. Some of what we have learned has been summarized in peer-reviewed publications such as articles in Nature Biotechnology, Nucleic Acid Therapeutics, and in a publication that in Trends in Molecular Medicines. These publications have been supplemented by numerous presentations at scientific meetings and podcasts like the interview that was just released by Rare New England’s Lisa Deck. We have also encountered unanticipated challenges, such as the need to have vials filled at a sterile fill facility rather than by hospital pharmacists and impacts of the extraordinary demand for n-Lorem ASOs. We have responded to these and other challenges and continue to make great progress. Nevertheless, the delays in treating some patients are concerning and we must work our way through the backlog of applications as rapidly as possible. This will mean that we must continue to grow our laboratory staff.
I am most pleased that we have established quality approaches at each step, greatly strengthened our team, completed the building of the needed infrastructure and are benefitting from specific FDA guidance specific for ASOs for nano-rare patients. It is also gratifying to hear about the positive experience that Dr. Neil Shneider has registered in patients with FUS ALS. However, we are disappointed at our inability to expand rapidly in response to the demand and that is our most important focus in 2022: deliver ASO treatments to n-Lorem patients, expand and work our way through the backlog of applications we have, so that we can respond in a timely fashion to new applications.
We thank all of the people who have joined our staff, our board and our Advisory Council, all our donors, large and small and our growing list of collaborators and partners. We could not have made the progress we have made without your support and the progress we make in the coming years will benefit from
your support as well.
Sincerely,

Stanley T. Crooke, M.D., Ph.D.
Dear colleagues, partners, and supporters,
I am pleased to have the opportunity to provide an update on the progress that we are making at n-Lorem. I hope that you agree that what we have accomplished to date is impressive, but more importantly sets the stage for bringing ever greater value to our patients.
Patient Treatment and Demand
As our mission is entirely focused on helping nano-rare patients, the single most important area of progress will always be patient-centered. I am pleased to tell you that Dr. Neil Shneider continues to report that the FUS ALS patients being treated with an experimental ASO continue to gain strength and improved function. The next step is, for us at n-Lorem, very exciting. We expect to initiate treatment of five to eight new patients before the end of 2022. Demand continues to be high. We have now received over 150 applications and have accepted 69 patients for treatment. One informative way to think of n-Lorem is that we are two-and-a-half-year-old biotechnology drug discovery effort with more than 60 drug discovery programs, more than 20 drugs in development, five to eight of which will begin clinical administration this year. Even though what we do at n-Lorem is very similar to what is done routinely in drug discovery and development at Ionis, each step in the process is sufficiently different that we have had to create entirely new systems and processes. Given all that, I think you can readily understand that our tiny core team has been stretched to our limits, but we are achieving great things and enjoying every moment of it.
Fundraising
Despite the successes we had in fundraising in 2020 and 2021, as we entered 2022, I felt that achieving our goal of raising $8M was going to be extremely challenging. Nevertheless, by the end of this month, we will have raised approximately $16M and given the numerous opportunities and leads that we have developed, I am optimistic that in the remaining months we will continue to be very successful in fundraising. The central reason for our success is our compelling mission. I am confident that we will enter 2023 with great momentum and an expanding base of donors, partners and supporters. To all those who have contributed, on behalf of the patients we serve, you have my deepest appreciation.
Organizational Development
Because of our success in fundraising, we have greatly accelerated our growth. We have established a senior leadership team that is truly outstanding, cohesive, committed and intensely demanding of ourselves and others. In recognition of her outstanding performance, the Board of Directors and I recently promoted Sarah Glass, Ph.D., to Chief Operating Officer. We are now filling in the next level in the leadership and management team and well along in completing the recruitment of a second laboratory. The second laboratory will be fully up and running by the end of this year and will double our capacity to advance the cases that we have accepted for treatment. I fully expect to be able to respond to each patient more rapidly and more effectively. Perhaps of even greater long-term importance, with the new lab and staff we are beginning to invest in core research that will address issues like being more efficient in discovering allele-specific ASOs.
Despite the demands for high-quality scientific and medical talent, we are thrilled with the quality of individuals who have chosen to join us. Once again, I am confident that our success here is driven by our mission.
FDA Interactions
Though we were very pleased that the FDA responded rapidly to provide detailed guidance specific for ASO experimental therapy for nano-rare patients that included many of our suggestions, we could not know how effective our interactions with individual divisions would be. Once again, at the risk of sounding like a broken record, the interactions with the divisions to date have been very supportive and the public posture of the FDA continues to be supportive.
Communications/Partner and Donor Relations
Since Amy Williford joined us the progress here has been wonderful. Our new presentation has been extremely well received, we have issued many positive press releases with more to come, our speaking engagements reflect the interest in having the n-Lorem model included in important discussions, and our contributions evidenced in the publication of important peer reviewed articles. Shortly, we will unveil a new website that will include better support to help patients find an appropriate center for diagnosis and treatment and support for institutions.
Quality Systems
It is vital that every step in the process be of the highest quality possible if we are to maximize the opportunity for benefit and minimize the risk of adverse events in our patients. We now have enough experience with our quality systems to be confident that they are working and that only appropriate patients are treated with the very best possible ASOs. We hope that some of organizations less experienced in ASO discovery and development adopt some of our quality systems.
Learning From Our Patients
I have adopted an expression first used by Thomas Jefferson to describe the Lewis and Clark Expedition. Jefferson referred to the expedition as the Corps of Discovery. To my mind n-Lorem in its own way is a corps of discovery for both the mind and the heart. Certainly, for me every day is an opportunity to learn intellectually and emotionally from our patients. To that end the approaches that we have developed to collect natural history data and to compare those data to results during treatment are critical to maximizing what we learn. We believe that our systems that are in place and are ready to maximize learning from each patient as we expand from five to eight patients this year, to many more next year and thousands in the decades to come, but as we gain experience, I am sure that we will continue to learn and refine our systems. In this effort, we are greatly aided by our partner, Parexel.
Partnerships
We continue to benefit from many partnerships we have already established with vendors that provide services to our industry, including manufacturing, sterile fill, GLP toxicology studies and clinical trial services. In our latest presentation, you will find a slide that lists our partners, each of whom is making significant contributions to our ability to treat more patients with higher quality. We have been quite successful this year in adding new partners and donors, and we are just getting underway. By the end of 2022, I expect that our roster of partners will be too large to show on a single slide. The response has been overwhelmingly supportive. Once again, the reason for the support is the mission. Everyone recognizes the gravity of the challenges that nano-rare patients face and the need for all of us to come together to create a community that can bring hope, help and benefit to these patients.
Patient Empowerment Program
We are very pleased to have launched this program with our podcast series. The goals of the podcast series are to provide a forum where the voices of patients, advocates and experts can come together focused on the nano-rare patient, and to help nano-rare patients and family to better understand why their lives are affected by these mutations and how challenging it is to address some of the problems via a series of didactic chats that go from the most basic to the complex task of understanding disease and creating treatments for the disease.
Conclusion
In this brief communication, I have shared a tiny bit of the progress that we are making. We are proud of the progress, but we understand that we have just started and the challenges that lie ahead must be met, and we must prove that a non-profit model is sustainable. We are buoyed by the progress that we have made and never more confident that with your help we can meet the challenges and prove that human hearts are big enough to address the needs of the nano-rare patient with a sustainable non-profit model.
Sincerely,

Stanley T. Crooke, M.D., Ph.D.
Dear n-Lorem Community,
In January of 2020, we announced the establishment of a non-profit foundation with the mission of providing experimental antisense oligonucleotide therapies (ASOs) to patients suffering from ultra-rare disease for free, for life. Despite the extraordinary adversity brought about by the Covid 19 pandemic, I am grateful that we have been able to make rapid progress in our efforts to serve n-of-1 patients. I invite you to discover our impact throughout the pages of the following report.
Meeting the Extraordinary Demand
When we founded n-Lorem, I imagined that patients would find us slowly and the number of applicants for treatment would be quite modest, but in parallel with establishing n-Lorem, we collaborated with two clinical investigators to treat 14 patients. Even more exciting is the growing demand. As of today, n-lorem has received 80+ patient applications, we have approved more than 30 patients for drug discovery and clinical development of a personalized medicine for each and now have more under review. Our first n-Lorem patients will soon receive treatments. To meet the demand, we have expanded rapidly, and I am thrilled with the quality of the team we have assembled as we discuss later. Many senior industry and academic leaders are donating their time. I think the reasons these busy eminent people are committed to n-Lorem can be summed up with this comment: “these desperate patients depend on us”.
Beginning in 2019 well before we formally initiated n-Lorem, we worked closely with the FDA and provided several proposals in response to the FDA’s request for public comment about guidance for approaches to the treatment of ultra-rare disease patients. We were therefore greatly encouraged and appreciative when the FDA provided guidance specific for ASO treatment of ultra-rare patients. The guidance was consistent with our recommendations and provides a critical set of directions on how to develop experimental ASOs. We continue to work with the FDA to add other elements of guidance if the FDA determines that even more specific guidance might be helpful. We are deeply appreciative of the rapid provision of guidance that addresses the unique challenges these patients present.
Establishing Quality Systems
Patients with ultra-rare diseases are typically complex and severely ill. It is vital that we do everything possible to assure that only the most appropriate patients are treated and that they are exposed to only prudent risks. (Sadly, no medicine is risk free). To help us make these complex judgements, the Access to Treatment Committee was created and is contributing enormorously to our successes. We must know the disease we are treating, why we are treating and that, if successful, the benefit will matter to the patient. Thus each patient has an individual treatment and natural history plan. We must assure that we provide every patient with the very best possible antisense medicine, and our collaboration with Ionis assures that. Finally, we must evaluate our performance and share what we learn with all stakeholders. The quality systems described here are unique and vital.
Establishing a Broad Network of Stakeholders and Contributors
We know that we cannot do this alone. We know that only via a network of all stakeholders can we maximize the value we can provide to our patients. We are, therefore, thrilled with all the stakeholders who have already joined us in our quest to change the futures of these patients and their families. Each of these collaborators and benefactors have also helped us spread the word that n-Lorem can offer immediate hope and rapid experimental treatment to ultra-rare diseases patients. In just 18 months, the contributions from many different types of stakeholders have exceeded $30 million in cash and significant in-kind donations. Without this generous support, we could not have met the remarkable demand we have experienced. We also believe that this start assures that we can raise the funds we need to meet the growing demand over the long term.
Our Vision
We have just begun, but we have an exhilarating long-term vision. As Jefferson said when the Lewis and Clark expedition was formed, we are the corps of discovery. We are pioneering an entirely novel approach to treating patients. We are creating a path and a model for others to follow and it is that leverage that will lead us from helping thousands of patients to millions. We can’t do it without you. On behalf of our patients and their families, I thank you. You have chosen to be a part of n-Lorem and, in the long run, that means you will be a contributor to more holistic solutions for these patients in the future.
With gratitude,

Stanley T. Crooke, M.D., Ph.D.

We cannot do
this alone
Together we are changing the world—
one patient at a time
We hope that you join us on this journey to discover, develop and provide individualized antisense medicines for free for life for nano-rare patients. The ultimate personalized medicine approach – for free, for life.
Follow us on social for updates on our latest efforts

