Grant References

Executives & Sr. Leaders
 Stanley CrookeFounder, Chairman of the Board & CEO
Stanley CrookeFounder, Chairman of the Board & CEO Sarah GlassChief Operating Officer
Sarah GlassChief Operating Officer Julie DouvilleExecutive Director ASO Discovery & Development
Julie DouvilleExecutive Director ASO Discovery & Development  Konstantia
Konstantia
Skourti-StathakiDirector ASO Design & Discovery
 Laurence MignonExecutive Director Clinical Development
Laurence MignonExecutive Director Clinical Development Cedrik NgongangSr. Director Medical Geneticist
Cedrik NgongangSr. Director Medical Geneticist
 Virginia SankeyExecutive Director Finance
Virginia SankeyExecutive Director Finance Megan KnutsenDirector Foundation & Project Management
Megan KnutsenDirector Foundation & Project Management
 Amy WillifordSr. Director Communication & Donor Relations
Amy WillifordSr. Director Communication & Donor Relations Kim ButlerSr. Director Operations
Kim ButlerSr. Director Operations Joseph Gleeson[Consultant] Part-time CMO
Joseph Gleeson[Consultant] Part-time CMO

Board of Directors
| NAME | TITLE | ORGANIZATION |
|---|---|---|
| Stanley Crooke, M.D., Ph.D. | Chairman and CEO of the Board of Directors | n-Lorem Foundation |
| Rosanne Crooke, Ph.D. | VP, Cardiovascular Disease | n-Lorem Foundation |
| Sarah Glass | Chief Operating Officer | n-Lorem Foundation |
| Lynne Parshall | Retired COO of Ionis | |
| Michael Hayden, M.D., Ph.D. | Killam Professor and Ionis Board of Directors member | University of British Columbia |
| Joseph Loscalzo, M.D., Ph.D. | Head of the Department of Medicine and Physician-in-Chief, Board of Directors member and Executive Committee member |
Brigham & Women’s Hospital, Harvard Medical School, Ionis and UDN |
| Frank Bennett | EVP & Chief Scientific Officer | Ionis Pharmaceuticals |
| Frank Sasanowski, M.S., M.P.H., J.D. | Director | Hyman, Phelps & McNamara, P.C. |
| Jason Wood | Founder and CEO | J. Wood Capital Advisors |
| Gene Yeo, MBA | Professor, Dept. of Cellular and Molecular Medicine | University of California San Diego |
| Amy McCooe | Executive Director | The Wolverine Foundation |
| Paul Compton | Global Head of Corporate & Investment Bank and President of Barclays Bank PLC (BBPLC) | Barclays Bank PLC (BBPLC) |
| Neil Shneider, M.D., Ph.D. | Claire Tow Associate Professor of Motor Neuron Disorders Director, The Eleanor and Lou Gehrig ALS Center |
Columbia University – Vagelos College of Physicians and Surgeons Department of Neurology and the Center for Motor Neuron Biology and Disease |
| Alan Lotvin, M.D | CEO & Co-Founder | Sequel Med Tech, LLC |
| Andrew Lo, PhD | Professor, Director and Principle Investigator | Laboratory for Financial Engineering (LFE) MIT Sloan School of Management |

Expert Review Committees and Organizations
| Name | Purpose |
|---|---|
| Access to Treatment Committee (ATTC) | To advise on whether a patient should be accepted for potential treatment. |
| Research Management Committee (RMC) | To assure that the ASO and treatment and evaluation plan are optimal. |
| FDA | To review each investigational new drug (IND) application and assure that the ASO and treatment plan meet standards. |
| Study Treatment and Assessment Review (STAR) | To assure the treatment plan is optimal. |
| Investigational Review Board (IRB) | Each medical institution has an independent review committee to assure ethical treatment of patients. |
| Data Safety Monitoring Board (DSMB) | To review all clinical safety data on a quarterly basis and determine whether safety profiles of the personalized ASOs are adequate. |

US FDA Guidance for ASO Medicines
for Nano-rare Patients
Issued ASO Guidance
- Initial FDA guidance for ASO for patients with diseases caused by ultra-ultra-rare mutations: Jan. 4, 2021
- Pre-clinical requirements: Detailed guidance April 2021
- CMC guidance Dec 2021
- Clinical guidance Dec 2021
- FDA response to n-Lorem concept supportive
- n-Lorem posed questions that require policy decisions, but progress toward policies evident
- In the meantime, experience facilitating ASOs for individuals provides real-life guidance

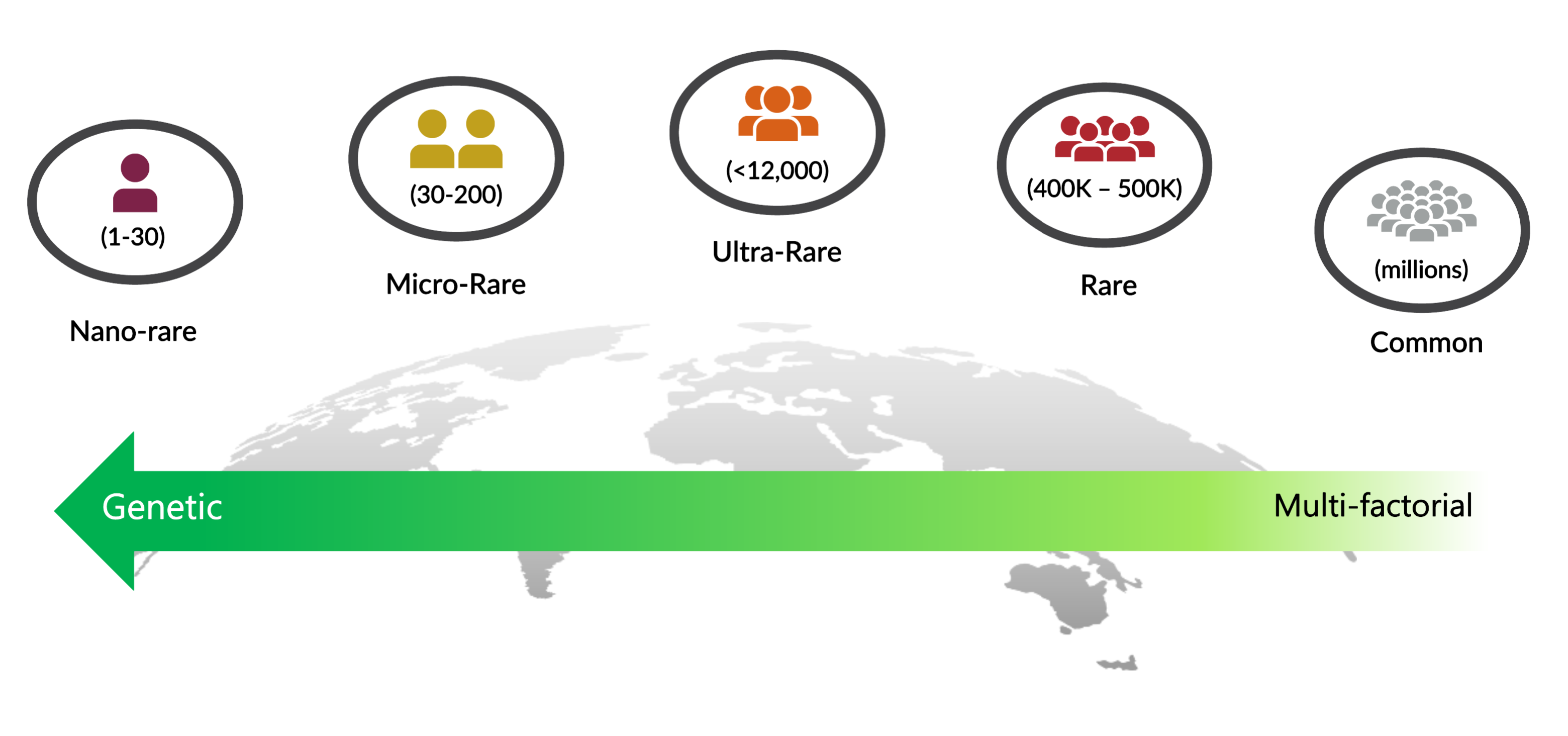
Nano-rare: 1 to 30 Patients Worldwide

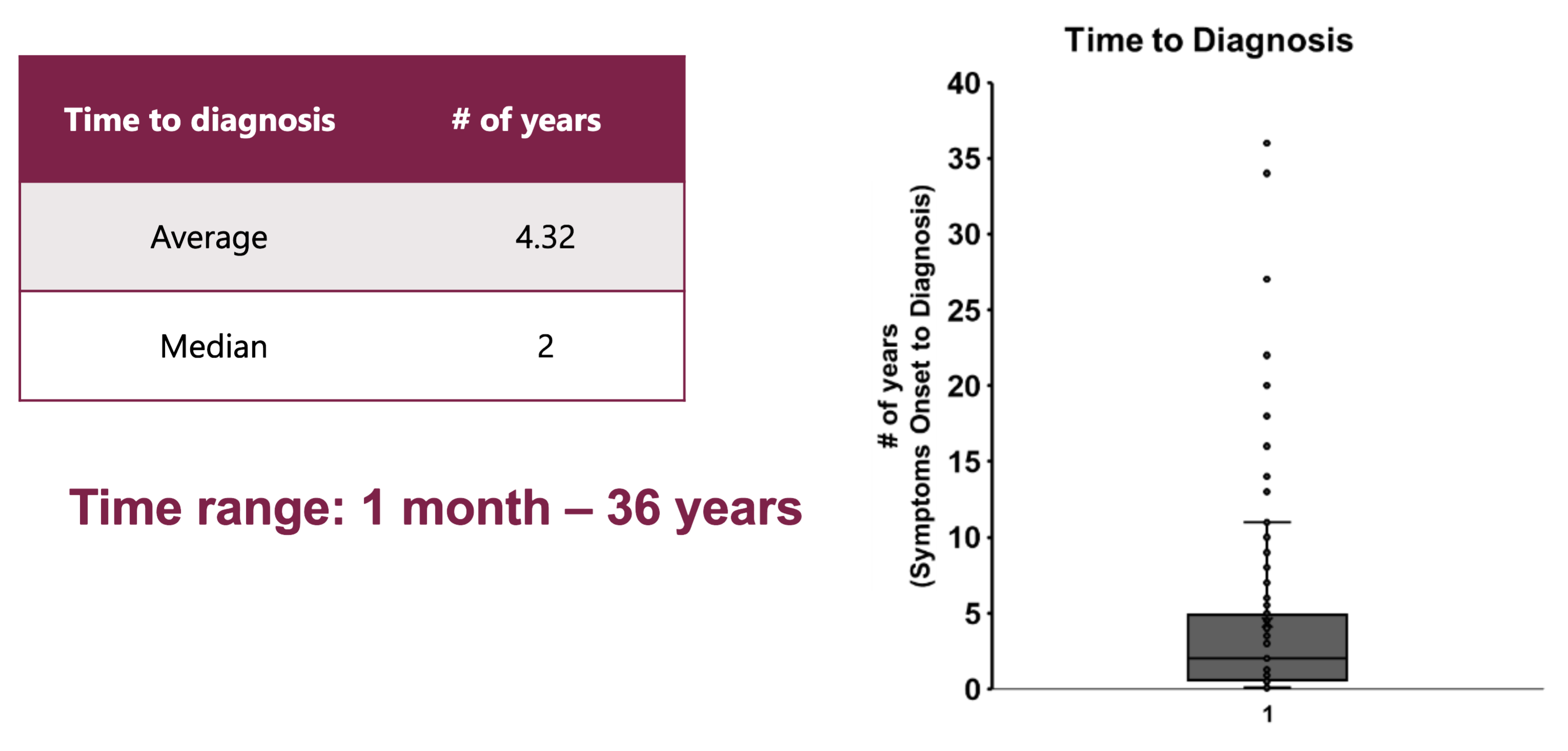
Significant Range in Time
from Symptom Onset to Diagnosis

SCN2A Patient: Mutation in the SCN2A gene
Known to exist in only 9 patients worldwide
History
- Hospitalized initially at one month
- First seizure at eight months
- hospice care at age 2
- 29 hospitalizations at 9 institutions
- More than 34 different diagnoses
- Treated with as many as 12 to 15 drugs at a time, but today untreated
Current Phenotype
- 13 years of age
- Modest seizure activity
- Severe movement disorder
- Developmental delays
- GI issue
Diagnostic and Treatment Odyssey
- Parents found and paid for an academic scientist to sequence patient
- Parents supported research to define the nature of the mutations and to demonstrate causality
- Many challenges in identifying an institution willing to consider an experimental treatment
- ASO treatment and evaluation

Quality Systems Established to Assure
the Best Outcomes and Max Learnings….

Patient Empowerment Program Podcast


Crooke, S.T., et.al., 173 patients. medRxiv 2024
https://medrxiv.org/cgi/content/short/2024.08.05.24310862v1
Genotypic and phenotypic analysis of 173 patients with extremely rare pathogenic
mutations who applied for experimental antisense oligonucleotide treatment
Stanley T. Crooke, Tracy A Cole, Jeffrey B Carroll, Joseph G Gleeson, Laurence Mignon, Julie Douville, Wendy Chung, Jennifer Bain, Elizabeth M Berry-Kravis, Nelson Leung, Scott Demarest, Emily McCourt, Andy Watt, Berit Powers, Cedrik Ngongang

Decades of Experience in
Creating and Advancing ASO Technology

Integrated Safety Databases Provide the Foundation
for Experienced Clinical Decision-Making

Leaders Across All Areas of Drug Discovery,
Development and Manufacturing Businesses Support
Treating Nano-rare Patients at n-Lorem
Biotech/Pharma Companies
Genomic Sequencing
Pre-clinical Toxicology CROs
Manufacturing
Clinical Management
Sterile Fill Product
Access to Appropriately Characterized
Patients and Investigators
Data Partners

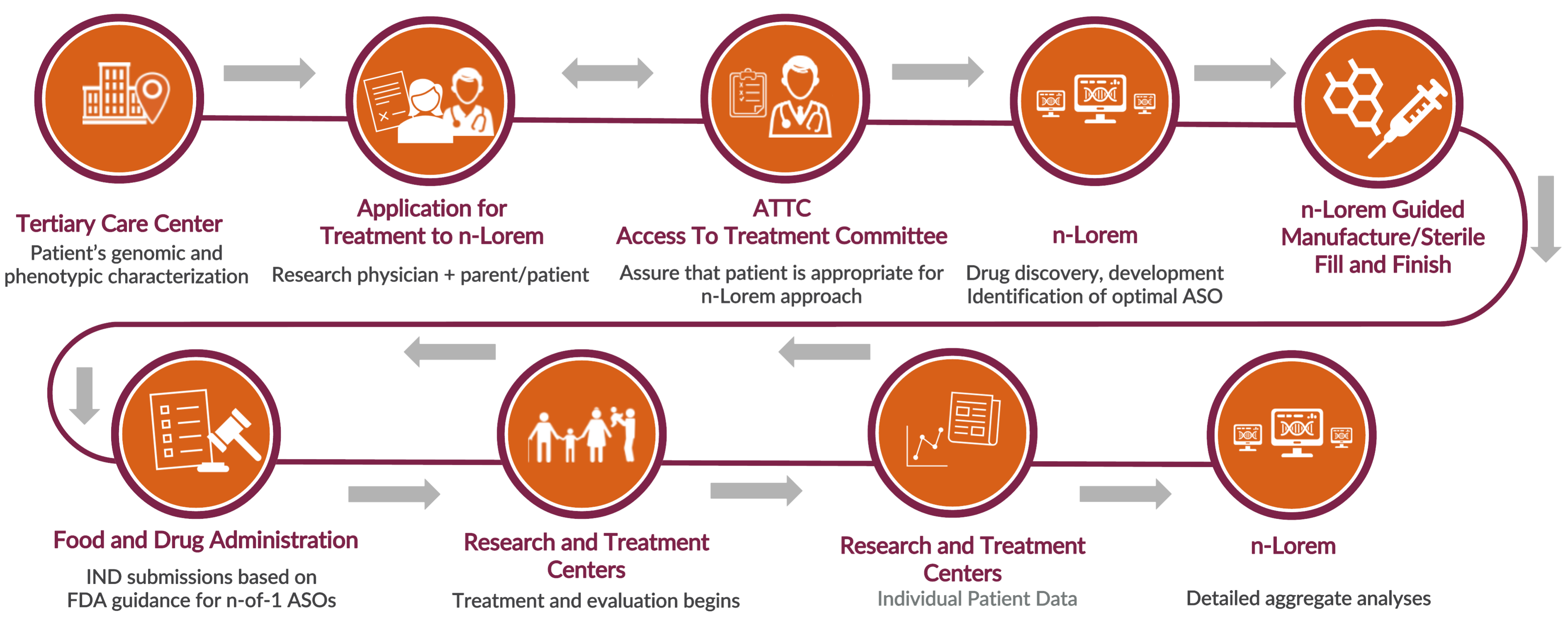
n-Lorem Research and Development Process
Current Treatment Institutions
- Children’s Hospital Colorado
- Columbia University
- Mayo Clinic – FL
- Mayo Clinic – MN
- New York University
- Rady Children’s Hospital
- Rush University Medical Center
Pending Treatment Institutions
- AdventHealth-Orlando
- Baylor College of Medicine
- Boston Children’s Hospital
- Cedars-Sinai Medical Center
- Children’s Healthcare of Atlanta
- Cleveland Clinic
- Cook Children’s Medical Center
- Dell Medical School
- Hackensack Meridian Health
- Hawaii Pacific Neuroscience
- Johns Hopkins University School of Medicine
- Massachusetts General Hospital
- Medical College of Wisconsin
- National Institute of Neurological Disorders and Stroke
- Northwestern University
- Phoenix Children’s Hospital
- Rutgers Robert Wood Johnson Medical School
- Seattle Children’s Hospital
- St. Jude Children’s Research Hospital
- The Hospital for Sick Children (SickKids)
- The University of Texas – Houston
- Thomas Jefferson
- University of California, San Diego
- University of Miami
- University of Michigan
- UT Southwestern
- Weill Cornell Medicine

Current n-Lorem Process To Discover and Develop
Optimal RNase H1 ASOs for CNS Disease
| Screening Step | Purpose | Approximate Minimum Numbers of ASOs Evaluated | Minimum Criteria |
|---|---|---|---|
| ASO design including in silico off-target assessment | Exclude motifs associated with ASO structure, repeat sequences, cytotoxicity, pro-inflammatory effects and off targets Include attractive motifs |
Scan entire pre-mRNA Apply algorithms |
All important attractive motifs included, unattractive excluded |
| Primary ASO screen | To identify optimal sites in target RNA for ASO and H-1 binding | ˜500 | >80% target reduction |
| Dose response evaluation of multiple ASOs | To select at least 20 ASOs for in vivo tolerability screening | ˜50-75 | IC50 1umol (free uptake) |
| In vitro off-target analysis | To confirm selectivity of ASO for target RNA vs. any worrisome off-target | As many as necessary | ˜10-fold difference in IC50s for target RNA vs. off-target |
| BJAB Assay | To exclude activators of innate immunity | ˜50-75 | Less than 2-fold increase in TNF-alpha at high ASO concentrations |
| Single dose tolerability screening in rodents at high dose including histopathology of CNS | To identify optimally tolerated lead ASOs | 20 | Exclude poorly tolerated candidate and include ASO with an optimal therapeutic index
AIF1 GFAP
Microglia Astrocytes |
| Repeat dose GLP 3-month rodent toxicity | To identify NOAEL and cell-types at risk | 1-3 | An attractive therapeutic index with an acceptable NOAEL |
| GMP Manufacturing | Quality ASO drug substance | 1 | Pure, stable drug product |
| Sterile Fill and Finish | Quality, stable and sterile ASO drug product | 1 | Sterile vials for administration |

Distribution of Submitted Cases

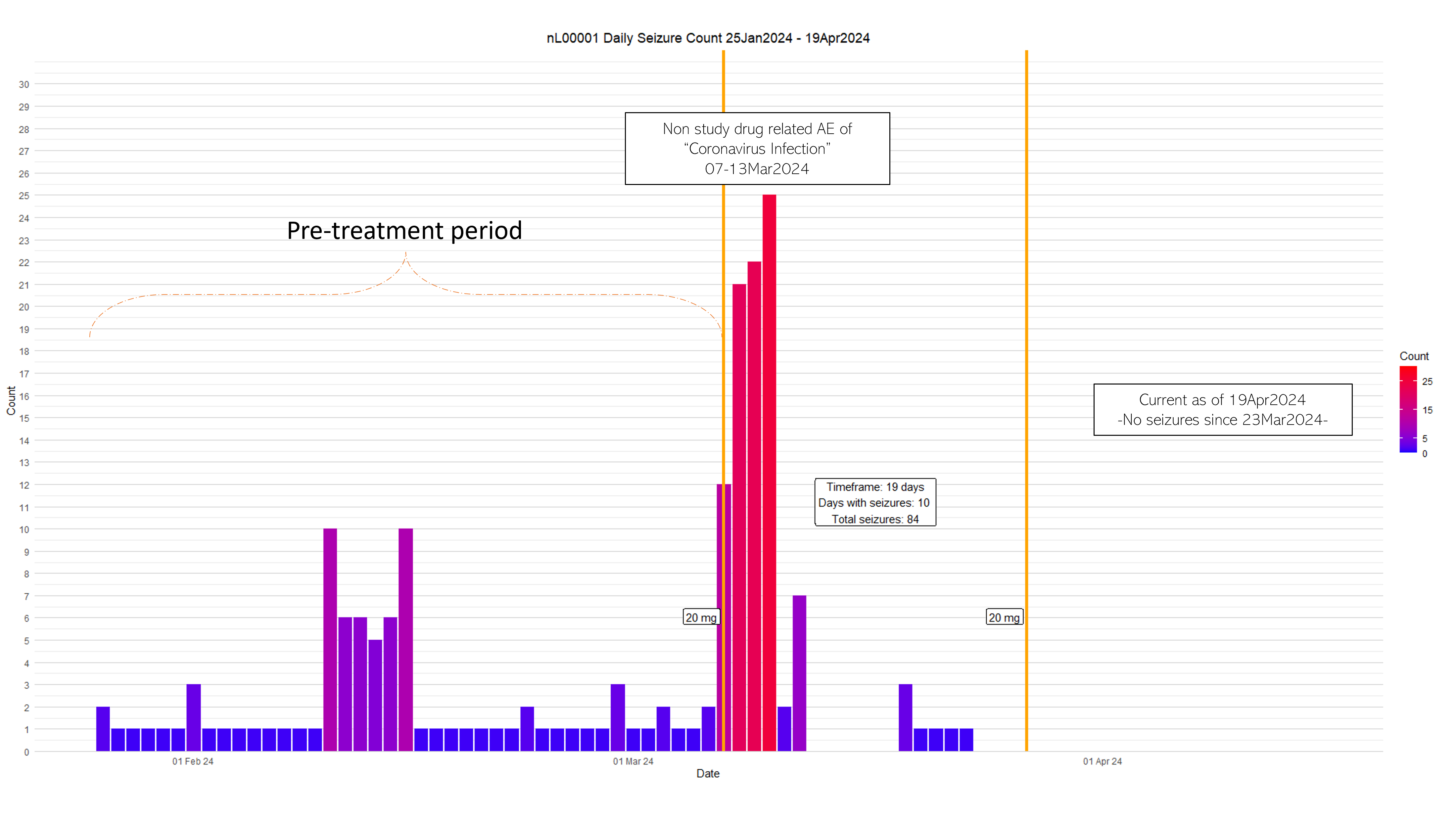
SCN2A Treated Nano-rare Patient
- First IT ASO dose nL-SCN2A-002 on 3/7 and second IT dose administered on 3/27 (20 days apart)
- Dose 3 on 5/22 (FDA CDER for dose escalation submitted on 4/19)
- No drug related AE or SAE
- Safety labs stable
- No seizures since 3/30 even in setting of upper respiratory viral infection
- had 21-25 seizures/day x3 days during Coronavirus infection (3/7) requiring emergency rescue seizure medication
- Baseline seizure frequency 1-2/night
- 3 lb. weight gain
- 4/8 typed “my brain feels calmer” on separate occasions
- Posted on ClinicalTrials.gov (NCT06314490)

SCN2A: Patient Seizures Before and After Treatment

Debilitating and Fatal Degenerative Disease Leaves
Little Hope for KIF1A Patients and Their Families
Prior to treatment, Susannah’s symptoms were severe and progressive:
- Global development delays
- Severe seizures (>100/day) unresponsive to common anti-epileptic medications
- Progressive spasticity of the lower limbs
- Painful chronic peripheral neuropathy
- Frequent falls resulting in multiple bone fractures
- Severely impaired walking and required wheelchair for mobility
- Communication only through simple sentences with frequent pauses
- Vision loss

KIF1A Patient: Clinical Improvements in
Multiple Domains, Including Seizures,
Falls, and Quality Of Life
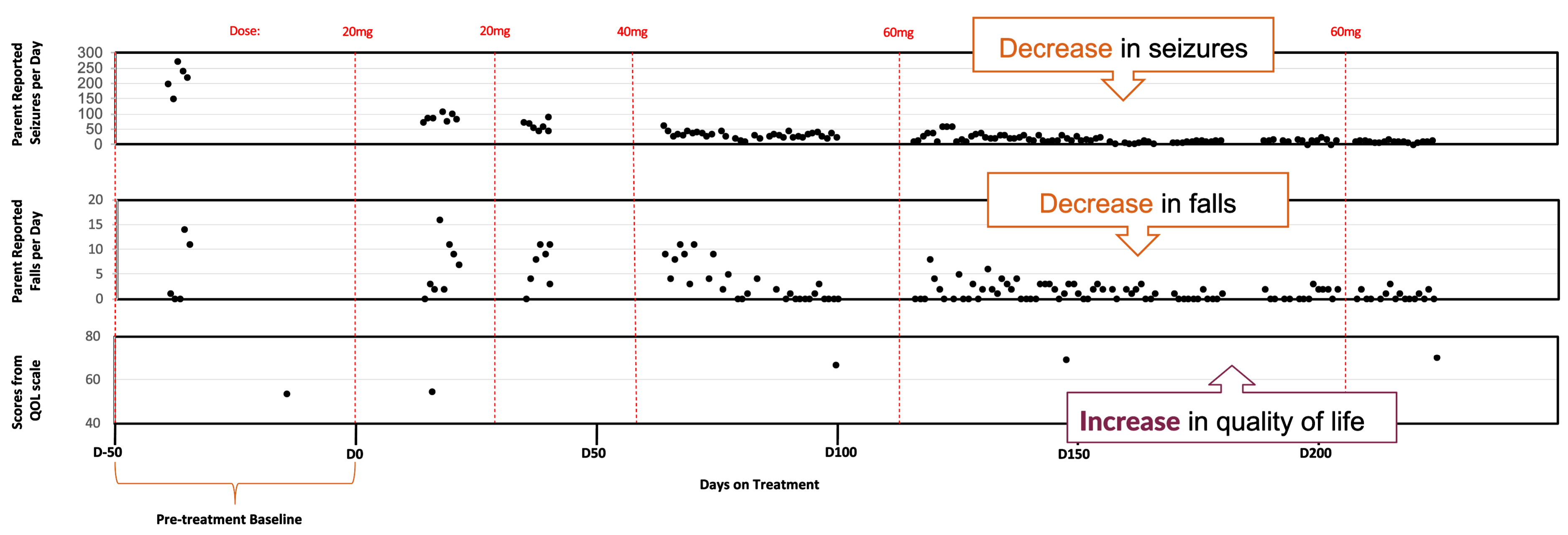
Clinical Evidence of Benefit Across Many Measures
No ASO-related Adverse Events

KIF1A Patient: Substantial Improvements Reported
Across Many Quality-of-Life Measures
| Measure | Baseline (before treatment) | Dose 1 | Dose 4 | Dose 7 |
|---|---|---|---|---|
| Alert and aware during the day | Sometimes | Sometimes | Often | Very Often |
| Been in a good mood | Sometimes | Sometimes | Often | Very Often |
| Showed happiness through body language | Often | Often | Very Often | Very Often |
| Expressed discomfort with changes in routine | Sometimes | Sometimes | Never | Rarely |
| Showed signs of being anxious or agitated | Often | Often | Sometimes | Rarely |
| Enjoyed moving their bodies (walking, etc) | Rarely | Rarely | Sometimes | Often |
| Expressed their needs (hunger, thirst) | Sometimes | Sometimes | Sometimes | Very Often |
| Enjoyed the social experience of mealtimes | Rarely | Rarely | Very Often | Often |
| Enjoyed spending time outdoors | Rarely | Rarely | Sometimes | Sometimes |
| Had enough energy to participate in daily routines | Sometimes | Sometimes | Sometimes | Often |
| Smiled or brightened their facial expression | Sometimes | Often | Often | Very Often |

KIF1A Patient: Significant Decrease in
Parent-Reported Pain Scores
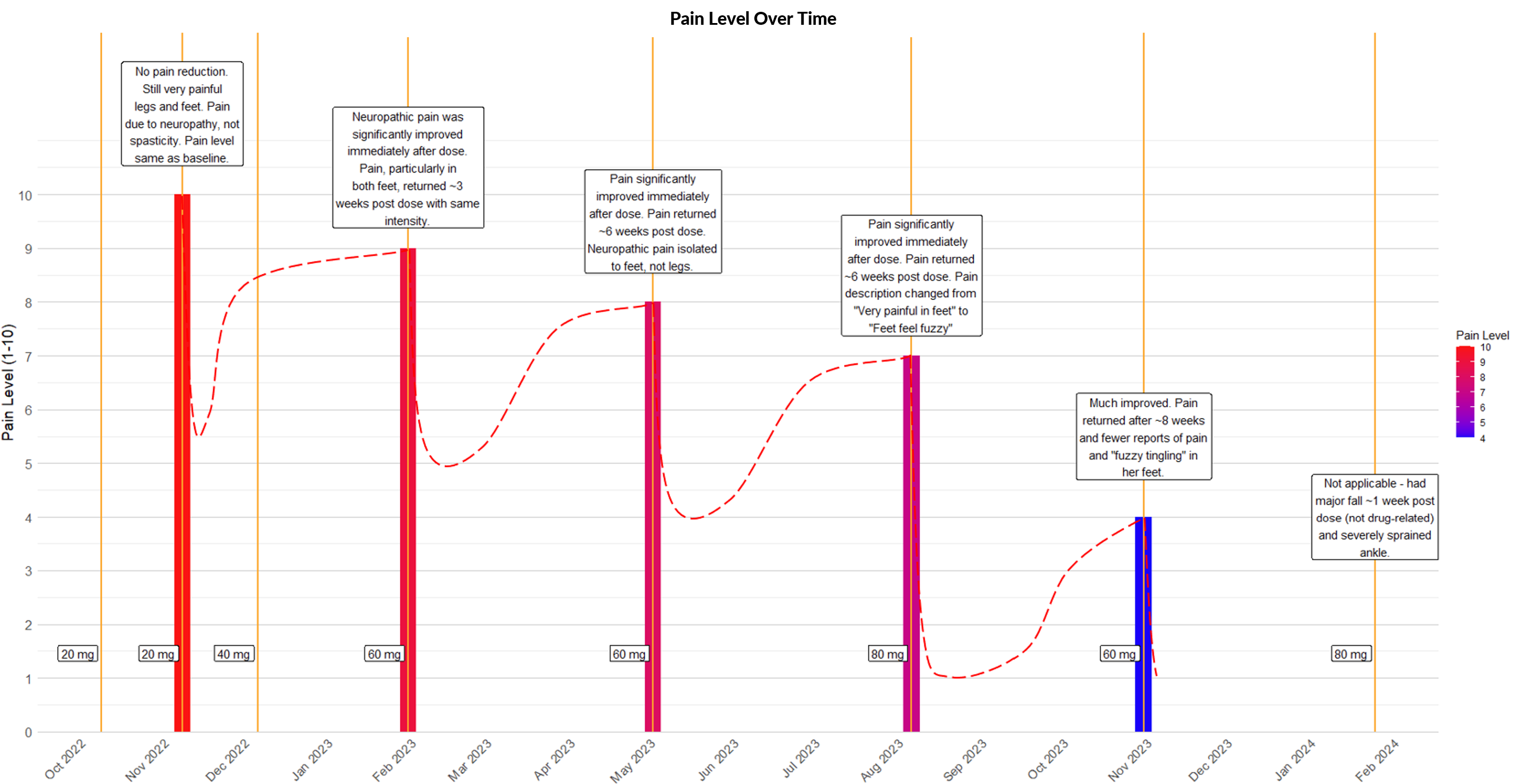
Clinical Evidence of Benefit Across Many Measures
No ASO-related Adverse Events

SAA Patient: Promising Evidence of Halting Amyloid
Progression Observed After Three Months of Treatment
- Stabilization observed across several markers of kidney function
Analyte Healthy Adult Values Baseline (before treatment) Month 1 Month 2 Month 3 Month 4 eGFR (mL/min/1.73m2) 55 63 60 56 60 Serum Albumin (g/dL) 3.5-5.0 4.2 4.1 4.2 4.3 4.0 BUN (mg/dL) 8-24 21 19 17 21 16 Serum Creatinine (mg/dL) 0.74-1.35 1.56 1.42 1.49 1.57 1.48 - Patient with progressive renal disfunction, prior to treatment was losing eGFR at a rate of 5-7 mL/min/1.73m2/year
- No drug related serious adverse events or adverse events

SAA Patient: Plasma SAA Levels at Baseline
(before) and During Treatment






